VOLUME 5 NUMBER 1 (January to June 2012)
Philipp. Sci. Lett. 2012 5 (1) 001-006
available online: January 13, 2013
*Corresponding author
Email Address: jose.isagani.janairo@dlsu.edu.ph
gerardo.janairo@dlsu.edu.ph
Submitted: November 24, 2011
Accepted: November 28, 2011
ARTICLE
Homology modelling and comparativedocking analysis of two naturally occurring pancreatic glucokinase mutants
by Jose Isagani B. Janairo1,3 and Gerardo C. Janairo2,3
1Physics Department, College of Science
2Chemistry Department, College of Science
3Center for Natural Sciences and Environmental Research (CENSER), College of Science
De La Salle University, 2401 Taft Avenue, Manila 1004, Philippines
2Chemistry Department, College of Science
3Center for Natural Sciences and Environmental Research (CENSER), College of Science
De La Salle University, 2401 Taft Avenue, Manila 1004, Philippines
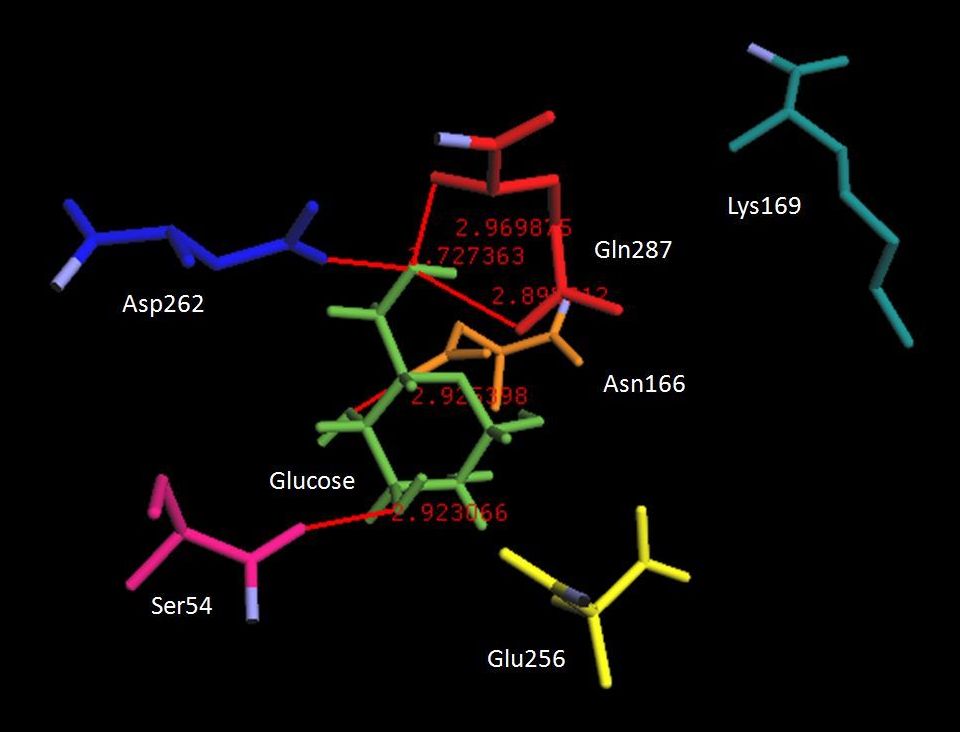
The homology models of two naturally occurringpancreatic glucokinase mutants with contrastingenzymatic behaviours were successfully generatedand accurately predicted. The homology modelsof the activated V367M and deactivated R369Pmutants were used for comparative docking analysis in order toprobe why such mutations led to either an increase ordiminishment of enzyme activity. Results of structuralcharacterization and docking simulations suggest that the smallconformational changes are responsible for the observedvariation in enzymatic activity. Iterative fitting and comparisonof their respective Ramachandran plots reveal that theseconformational changes are not sufficient to perturb the overallprotein architecture. However, active site modelling showed thatthese conformational changes altered the manner of ligandbinding, from which the observed contrasting enzymaticbehaviours originate.
© 2024 SciEnggJ
Philippine-American Academy of Science and Engineering