VOLUME 16 NUMBER 2 (July to December 2023)
SciEnggJ. 2023 16 (2) 359-366
available online: October 23, 2023
*Corresponding author
Email Address: aayanos@up.edu.ph
Date received: March 7, 2023
Date revised: June 17, 2023
Date accepted: August 21, 2023
DOI: https://doi.org/10.54645/2023162DHC-47
ARTICLE
β-Mannanase from Bifidobacterium adolescentis DSM 20083: Molecular Cloning, Expression, and Biochemical Characterization for Manno-Oligosaccharides Production Using Makapuno
Philippines Los Baños, College, Laguna 4031, Philippines
2Food Biotechnology Laboratory, Department of Food Science and
Technology, BOKU - University of Natural Resources and Life Sciences
Vienna, Austria
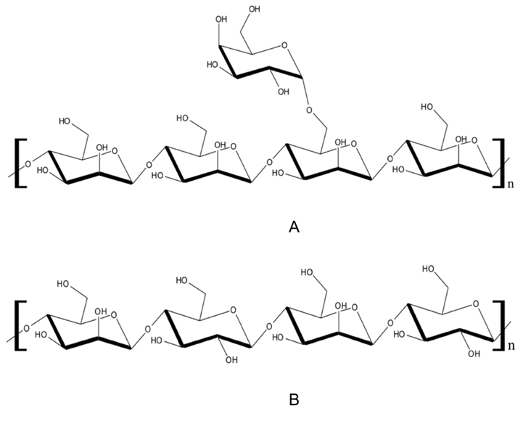
β-Mannanase has gained increasing interest recently due to its ability to degrade mannan polymers and produce high-value products such as manno-oligosaccharides (MOS). In this work, the tendency of β-mannanase from a human isolate, Bifidobacterium adolescentis DSM 20083, to produce potentially prebiotic MOS by hydrolyzing mannan-rich agricultural substrates such as makapuno (Cocos nucifera L.), a naturally occurring coconut variant, was investigated. A truncated variant of the β-mannanase gene was successfully cloned and heterologously expressed in Escherichia coli BL21(DE3). In SDS-PAGE, the recombinant β-mannanase was apparently homogeneous and had a molecular mass of about 110 kDa. The purified enzyme was found to have a specific activity of 66.1 Uman/mg when locust bean gum was used as substrate. The optimum temperature and pH of activity for the enzyme were obtained at 37 °C and pH 5.3, respectively. Among the cations tested, Co2+ was found to increase enzyme activity by 63%. Kinetic measurements showed Km, vmax, kcat and kcat/Km values of 0.32 ± 0.03 mg/mL, 42.4 ± 1.2 µmol/min-mg, 71.3 ± 1.9 /s and 221 ± 30 mL/mg-s, respectively. Analysis using HPAEC-PAD indicated that using makapuno, the major hydrolysis products of the enzyme are 61-α-D-galactopyranosyl-β-1,4-mannobiose (107 mg/g) and 61-α-D-galactopyranosyl-β-1,4-mannotriose (68.1 mg/g) amounting to 88% of total MOS produced. This study has shown that the β-mannanase from B. adolescentis could be used to produce MOS which could be of interest to the food industry.
© 2026 SciEnggJ
Philippine-American Academy of Science and Engineering