VOLUME 16 NUMBER 2 (July to December 2023)
SciEnggJ. 2023 16 (2) 392-402
available online: November 30, 2023
*Corresponding author
Email Address: nsquiming@up.edu.ph
Date received: July 18, 2023
Date revised: October 12, 2023
Date accepted: November 6, 2023
DOI: https://doi.org/10.54645/2023162NQS-33
ARTICLE
High-Performance Liquid Chromatography (HPLC) Method Validation for Simultaneous Quantitation of Five Phytoestrogenic Flavonoids
Sciences, University of the Philippines Manila, Manila, Philippines
2Regenerative Biology Research Laboratory, Institute of Biology, University
of the Philippines Diliman, Quezon City, Philippines
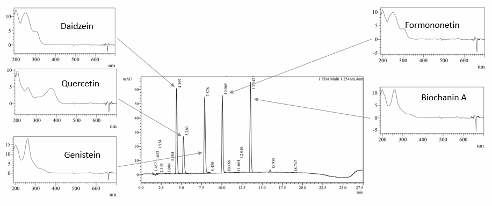
A Reversed Phase High-Performance Liquid Chromatography (RP-HPLC) method for simultaneous quantitation of five phytoestrogens namely daidzein, genistein, formononetin, and biochanin A, and quercetin was developed and validated through the evaluation of linearity, accuracy, precision, specificity, limit of detection and limit of quantitation in accordance with the ICH guidelines. The analysis was performed in a C18 column (150 x 4.6 mm, 5 µm) with optimized gradient elution using acetonitrile-water (0.1% trifluoroacetic acid) as mobile phase at a flow rate of 1.0 mL min-1 and sample injection volume of 10 mL. The retention times of the standards daidzein, quercetin, genistein, formononetin, and biochanin A were 4.42, 5.24, 7.85, 10.06, and 13.55 min, respectively with tailing factors ranging from 1.09 to 1.12 and a minimum resolution value of 3.74. Detection limits ranged from 0.339 to 0.964 ug/mL and quantitation limits ranged from 1.027 to 2.922 µg/mL with good linearity (R2 ≥ 0.9967) within 1.25 to 20 µg/mL concentrations of the standards. The method was also found to be accurate and precise based on percentage recovery ranging from 96.96% to 106.87% (intraday, n=3) and relative standard deviation of %RSD≤1.45% (intra-day, n=3) and %RSD≤2.35% (inter-day, n=5). The specificity of the method was evaluated based on the positivity of the minimum peak purity index during the quantitation of the target compounds from the spiked hydrolyzed and unhydrolyzed extract of Cajanus cajan ICPL 7035.
© 2026 SciEnggJ
Philippine-American Academy of Science and Engineering