VOLUME 16 (Supplement)
SciEnggJ 16 (Supplement) 058-066
available online: August 29, 2023
DOI: https://doi.org/10.54645/OKCW42826
*Corresponding author
Email Address: alvillaraza@up.edu.ph
Date received: March 31, 2023
Date revised: July 28, 2023
Date accepted: August 14, 2023
ARTICLE
On-resin synthesis of the somatostatin venom analog Consomatin Ro1
Diliman, Quezon City, 1101, Philippines
2Marine Science Institute, College of Science,
University of the Philippines, Diliman, Quezon City, 1101, Philippines
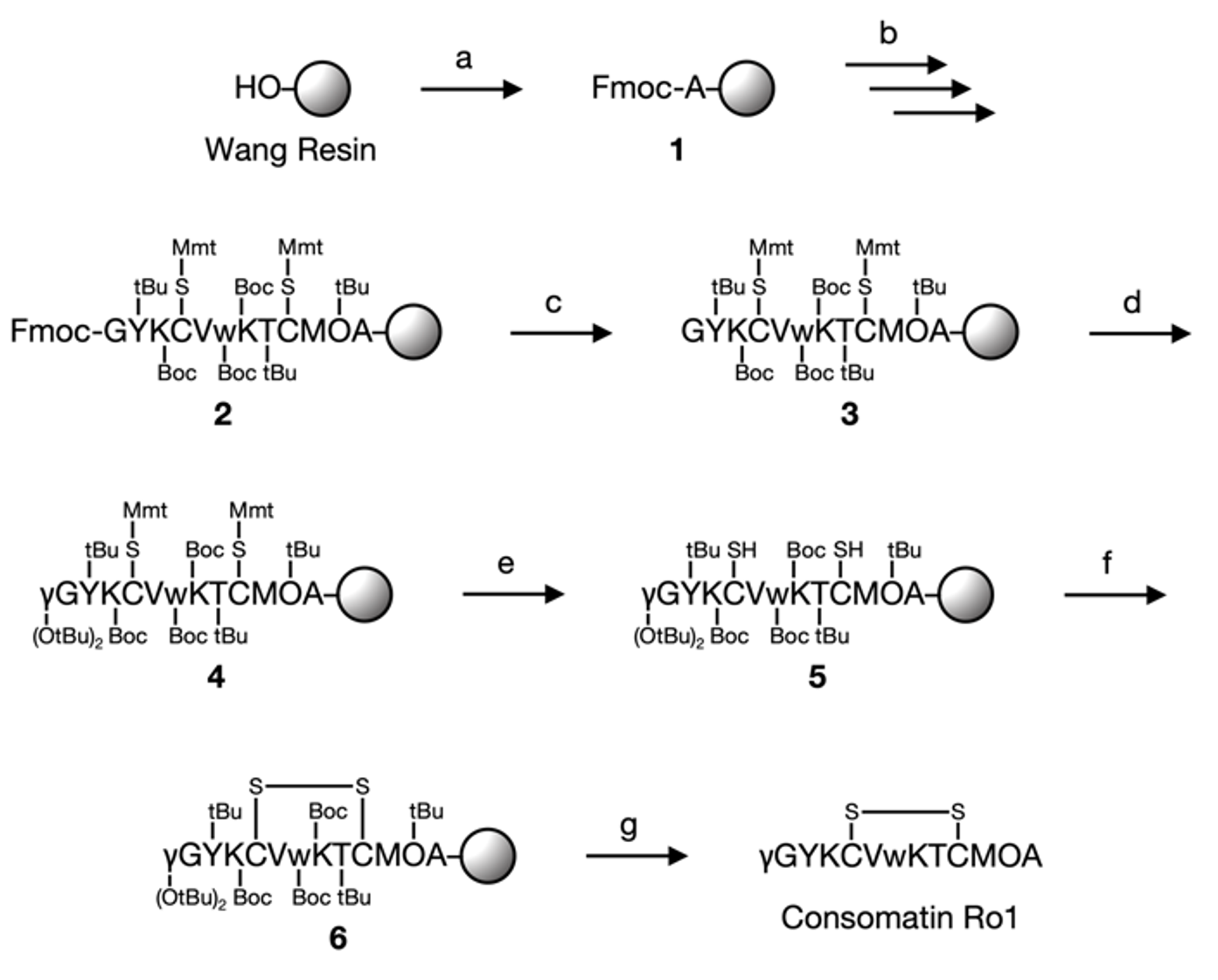
Consomatin Ro1 is a disulfide-containing peptide derived from the venom of the cone snail Conus rolani with a sequence that is similar to the vertebrate peptide hormone somatostatin. It has been shown to preferentially activate human somatostatin receptor subtypes 1 and 4, and to exhibit antinociceptive and antihyperalgesic properties making it an interesting peptide to study and develop as a chemical probe or an analgesic drug. Here, we describe the synthesis of Consomatin Ro1 using an on-resin approach wherein the disulfide bond is formed while the peptide is still attached to the resin. This was achieved by selectively removing the methoxytrityl protecting group of Cys residues with a weak acidic mixture, and treating the resulting thiol-containing peptidyl resin with the mild oxidant N-chlorosuccinimide. The strategy yielded a considerably higher amount of the peptide when compared with the previously reported in-solution disulfide formation method.
© 2024 SciEnggJ
Philippine-American Academy of Science and Engineering