VOLUME 17 (Supplement)
SciEnggJ 17 (Supplement) 425-431
available online: October 31, 2024
DOI: https://doi.org/10.54645/202417SupEWZ-61
*Corresponding author
Email Address: syrinefaithdino@gmail.com
Date received: 08 January 2024
Date revised: 30 June 2024
Date accepted: 28 October 2024
ARTICLE
Optimization of immediate-release cilostazol tablets using Quality by Design (QbD) approach
Metro Manila 1000, Philippines
2Graduate School, Centro Escolar University, San Miguel, Manila,
Metro Manila 1008, Philippines
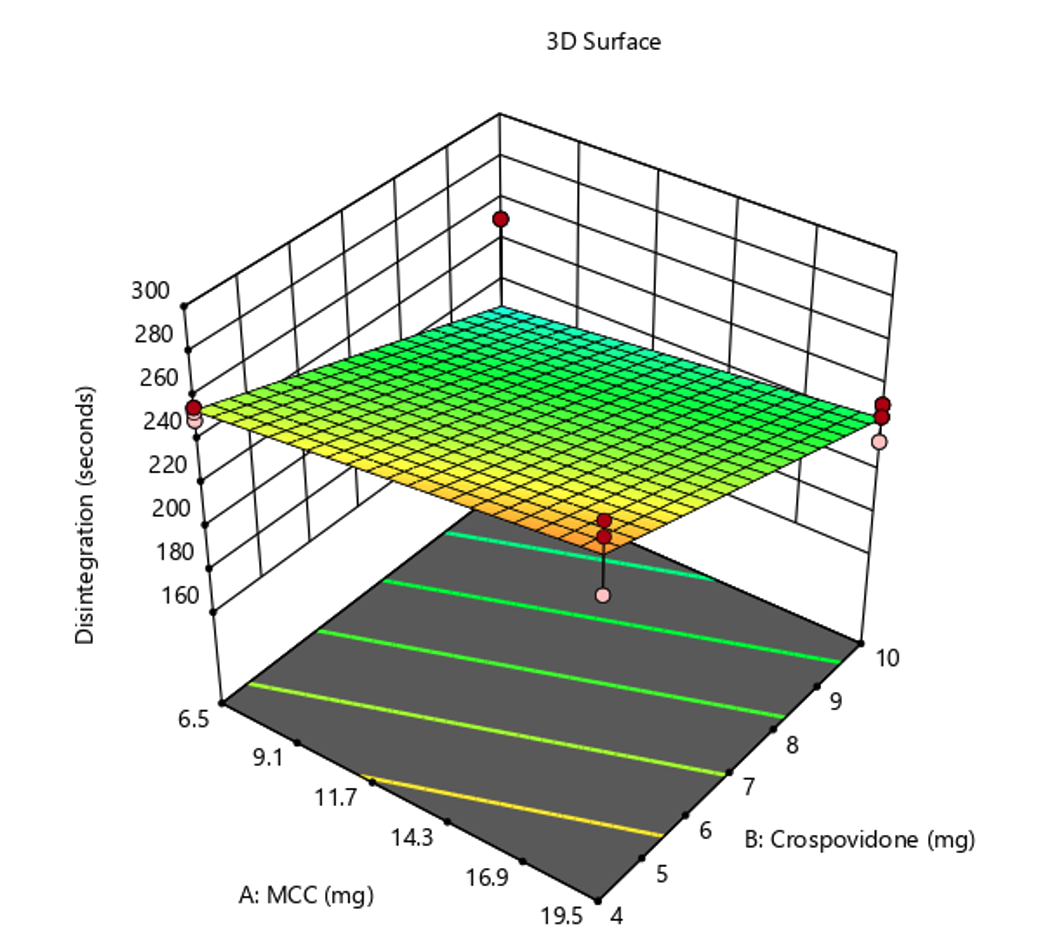
Cilostazol, recognized for its antiplatelet and vasodilatory properties, serves as a mainstay therapeutic agent in clinical management, targeting the alleviation of intermittent claudication associated with peripheral arterial disease. Despite its clinical efficacy, the pharmacological utility of cilostazol is delayed by its suboptimal absorption kinetics within the gastrointestinal tract. Given this limitation, the present study aimed to develop an optimized formulation of immediate-release (IR) cilostazol tablets employing the principles of Quality by Design (QbD). The general objective was to enhance the gastrointestinal absorption profile of cilostazol using combined disintegrants. The study investigated several parameters to develop an optimized formulation. The evaluation encompassed the micromeritic properties of the powder, quality control evaluations of tablet characteristics, and the identification of critical quality attributes (CQAs). Among the four trial formulations investigated, the third formulation, characterized by a low and high concentration ratio of microcrystalline cellulose (MCC) and crospovidone, emerged as the most promising. Notably, this formulation exhibited favorable results across all evaluated parameters, particularly demonstrating significant improvements in disintegration time. The observed enhancement in cilostazol's solubility suggests a synergistic effect provided by the addition of both MCC and crospovidone as disintegrants in the formulation.
© 2025 SciEnggJ
Philippine-American Academy of Science and Engineering