VOLUME 17 (Supplement)
SciEnggJ 17 (Supplement) 042-088
available online: March 18, 2024
DOI: https://doi.org/10.54645/202417SupJEA-58
*Corresponding author
Email Address: lsreyes@msi.upd.edu.ph
Date received: August 30, 2023
Date revised: November 29, 2023
Date accepted: January 10, 2024
ARTICLE
Synthesis and biological evaluation of cyanobacterial-inspired peptides
Diliman, Quezon City, 1101 Philippines
2Institute of Chemistry, College of Science, University of the Philippines,
Diliman, Quezon City, 1101 Philippines
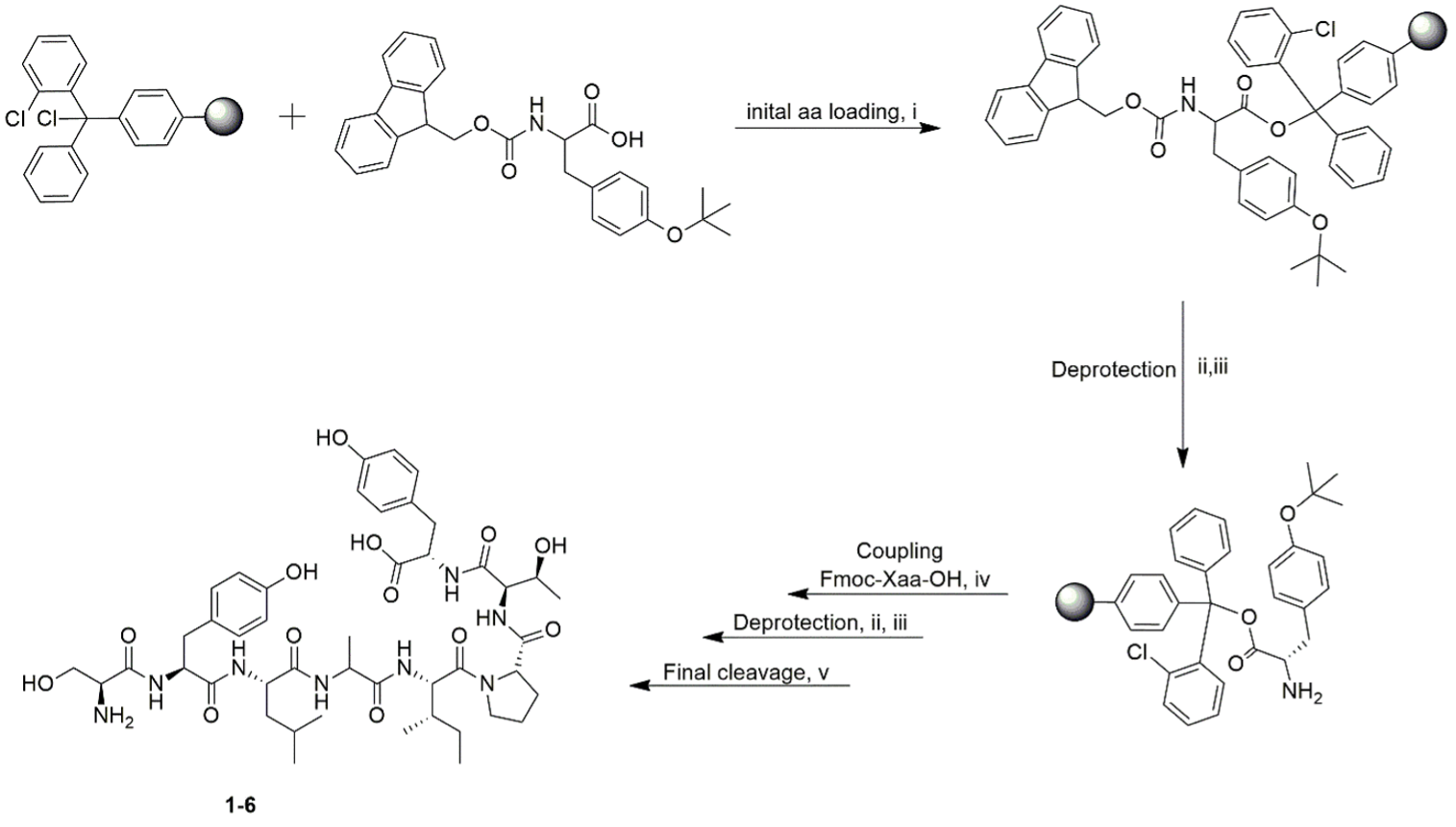
Cyanobacteria are known producers of structurally diverse and potent natural products; the majority are peptides with unique modifications. Yet, there remains a huge underexplored chemodiversity from cyanobacteria. Here, we designed a linear octapeptide as a product of combinatorial peptide design inspired by the natural products from the filamentous cyanobacteria Hapalosiphon welwitschii and Leptolyngbya sp. The target peptide was synthesized via solid-phase peptide synthesis (SPPS) using fluorenylmethyloxycarbonyl-protecting group (Fmoc) strategy. Structural diversity was expanded by the substitution of unnatural amino acids to yield five analogues. The structure and sequence of the synthesized peptides were confirmed using nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). Biological activity evaluation was done; with none of the peptides showing antimicrobial or cytotoxic activities against microbial pathogens and mammalian cells, respectively. To our knowledge, this study is the first to report a combinatorial peptide design inspired by a natural product and a predicted biosynthetic product. This strategy of peptide design expands the chemistry of a known bioactive natural product with the aid of unexplored cyanobacterial biosynthetic gene clusters.
© 2024 SciEnggJ
Philippine-American Academy of Science and Engineering