VOLUME 18 (Supplement)
SciEnggJ 18 (Supplement) 256-269
available online: 30 July 2025
DOI: https://doi.org/10.54645/202518SupDSK-64
*Corresponding author
Email Address: ahulog@ateneo.edu
Date received: 27 December 2024
Dates revised: 29 January 2025; 02 June 2025
Date accepted: 06 July 2025
ARTICLE
Development of a microfluidic platform for the encapsulation of baker’s yeast Saccharomyces cerevisiae in alginate hydrogel microparticles
Science and Engineering, Ateneo de Manila University,
Katipunan Avenue, 1108 Quezon City, Philippines
2Department of Chemistry, School of Science and Engineering,
Ateneo de Manila University, Katipunan Avenue, 1108
Quezon City, Philippines
3Department of Physics, School of Science and Engineering,
Ateneo de Manila University, Katipunan Avenue, 1108
Quezon City, Philippines
4Department of Electronics, Computer, and Communications
Engineering, School of Science and Engineering, Ateneo de
Manila University, Katipunan Avenue, Quezon City, 1108,
Philippines
5Department of Mechanical Engineering, College of Engineering,
University of the Philippines, Diliman, Quezon City, 1101,
Philippines
¶These authors contributed equally to this manuscript.
The encapsulation of cells in a semi-permeable polymer matrix enables the simulation of biochemical microenvironments for studying cellular response and interactions with minimal reagent use. Traditional methods often lack reproducibility, leading to variations in encapsulation quality. In contrast, microfluidic techniques can rapidly and consistently produce hydrogel microstructures encapsulating living cells. However, the fabrication of these devices remains challenging due to high initial costs and facility requirements. To address this, a microfluidic platform was developed using a CNC milling machine to enhance the accessibility of microfluidic-based cell encapsulation in hydrogel microstructures.
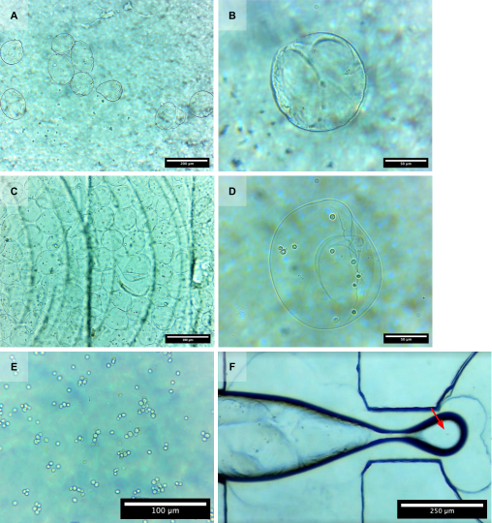
A flow-focusing droplet generator was designed and employed to generate alginate-in-oil droplets containing baker’s yeast cells. The microfluidic device was fabricated from PMMA using CNC milling and sealed via solvent-assisted bonding, ensuring accessibility and ease of production compared to traditional lithography-based fabrication methods. The optimized system operated at a 400:4 oil-to-alginate phase flow rate, generating monodisperse alginate droplets at 10 Hz. The droplets were subsequently gelated in calcium chloride to form alginate microparticles. The resulting microparticles, averaging 164.14 ± 15.11 μm in the Feret diameter along the major axis, exhibited controlled morphology, with predominantly teardrop and oval shapes. Imaging confirmed the presence of multiple spherical-ovoid yeast cells within the resulting hydrogels, verifying successful encapsulation. The findings support the feasibility of using CNC-milled microfluidic platforms for controlled and reproducible yeast encapsulation in hydrogel matrices, demonstrating their potential for broader biological and biotechnological applications.
© 2026 SciEnggJ
Philippine-American Academy of Science and Engineering