VOLUME 18 (Supplement)
SciEnggJ 18 (Supplement) 311-324
available online: 17 September 2025
DOI: https://doi.org/10.54645/202518SupHXQ-22
*Corresponding author
Email Address: jparreno@udel.edu
Date received: 29 June 2025
Date revised: 14 August 2025
Date accepted: 25 August 2025
ARTICLE
Scaffold-free generation of non-contractile bioengineered cartilage to investigate the effects of inflammatory mediators on human chondrocytes
University of Delaware, Newark, DE
2Department of Orthopaedic Surgery, Nemours Children’s Hospital,
Delaware, Wilmington, DE
3Department of Biomedical Engineering, College of Engineering,
University of Delaware, Newark, DE
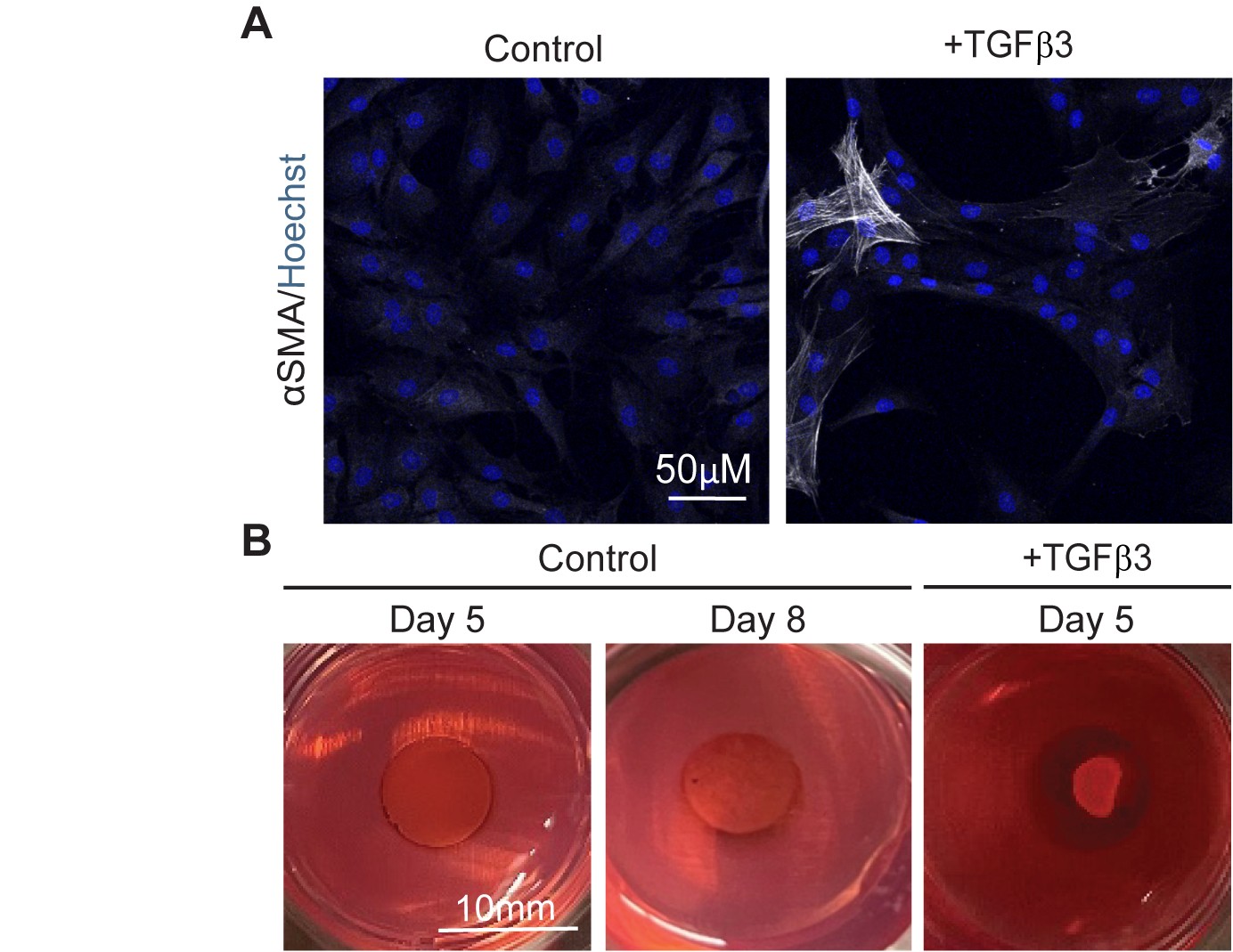
Current animal models, particularly in rodents, offer insight into Osteoarthritis mechanisms but translational limitations, due to species specific differences with humans, exist. Human cell culture models can be used, however, cell culture on monolayer may not fully replicate the molecular context of native human chondrocytes within a cartilaginous matrix. To address these limitations, we sought to generate bioengineered human cartilage tissues using passaged chondrocytes. To achieve this, passaged human chondrocytes were seeded within three-dimensional (3D) agarose molds and stimulated to redifferentiate by exposure to TGF-β3. While TGF-β3 upregulates cartilage-specific matrix molecule aggrecan (ACAN) and type II collagen (COL2A1) mRNA levels, TGF-β3 also enhances the contractile phenotype of cells which led to tissue shrinkage. To prevent tissue shrinkage, we targeted actin stress fibers by supplementing media with actin polymerization inhibitor, latrunculin A, which prevented contraction for up to 10 days. To prevent contraction after 10 days, we treated cells with Rho-associated coiled-coil kinase (ROCK) pathway inhibitor, Y-27632. This combination effectively reduced passaged cell mediated contraction preserving tissue integrity. Next, to determine if cytokine treatment led to cartilage degradation in bioengineered constructs, we exposed tissues to the inflammatory cytokine, interleukin-1 beta (IL1β). IL-1β reduced chondrogenic molecule and increased expression of matrix-degrading enzyme mRNA levels. These mRNA level alterations were accompanied by diminished ACAN staining and increases in staining for matrix metalloproteases-13, indicating a shift toward a matrix catabolism. Non-contractile bioengineered cartilage may be suitable to investigate the response of human chondrocytes to inflammatory mediators to provide further insights into human OA progression.
© 2026 SciEnggJ
Philippine-American Academy of Science and Engineering