VOLUME 18 (Supplement)
SciEnggJ 18 (Supplement) 380-385
available online: 24 November 2025
DOI: https://doi.org/10.54645/202518SupTYW-89
*Corresponding author
Email Address: jccasauay@up.edu.ph
Date received: 24 September 2025
Date revised: 30 June 2025
Date accepted: 19 September 2025
ARTICLE
Development and validation of an HPLC-PDA method for the quantification of fluconazole and application to fluconazole-loaded buccoadhesive film
University of the Philippines Manila, Ermita, Manila, 1000,
Philippines
2Department of Industrial Pharmacy, College of Pharmacy,
University of the Philippines Manila, Ermita, Manila, 1000,
Philippines
3Laboratory of Materials, Department of Physical Sciences and
Mathematics, College of Arts and Sciences, University of the
Philippines Manila, Ermita, Manila, 1000, Philippines
4Department of Clinical, Social and Administrative Pharmacy,
College of Pharmacy, University of the Philippines Manila,
Ermita, Manila, 1000, Philippines
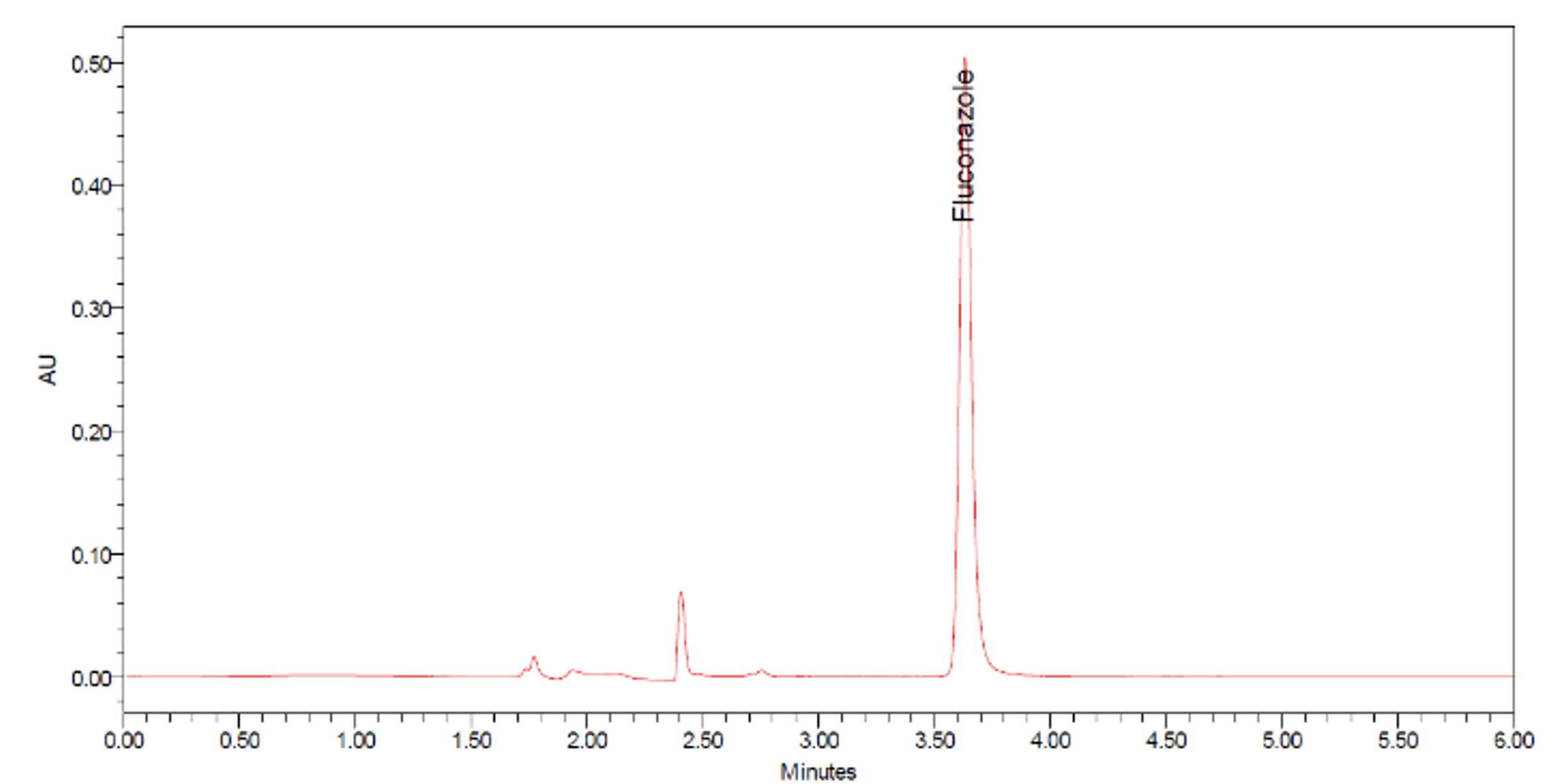
Fluconazole is a widely used antifungal drug against Candida infections, including oral candidiasis. To enhance its bioavailability for oral candidiasis management, buccoadhesive dosage forms are being suggested as alternatives to oral dosage forms. This study aimed to develop a simple and reliable high-performance liquid chromatography-photodiode array (HPLC-PDA) method for the detection and quantification of fluconazole in buccoadhesive film formulation. Chromatographic analysis was performed using C18 column (250 mm x 4.6 mm, 5 μm particle size, 100 Å pore size) under isocratic conditions, with mobile phase consisting of water and acetonitrile at a ratio of 75:25. The method used a 10 μL injection volume, a flow rate of 1.20 mL/min, detection at 257 nm under ambient temperature of around 25°C and was run for 6 min. The fluconazole peak appeared at 3.655 ± 0.019 min. The number of theoretical plates was 28238.43 ± 301.34, and the tailing factor was 1.31 ± 0.03, both meeting the acceptable criteria. Method validation confirmed linearity with a coefficient of determination (r2) of 0.9999, good accuracy with a percentage recovery range of 96.28 - 103.57%, and good precision with a percent coefficient of variation below 15%. The acceptable accuracy and precision were also observed when the method was applied in the assay of fluconazole incorporated in buccoadhesive film. Based on the results, the developed HPLC-PDA method proved to be suitable for the detection and quantification of fluconazole in buccoadhesive film formulation.
© 2026 SciEnggJ
Philippine-American Academy of Science and Engineering