VOLUME 6 NUMBER 1 (January to June 2013)
Philipp. Sci. Lett. 2013 6 (1) 090-096
available online: April 19, 2013
*Corresponding author
Email Address: alanx3@yahoo.com
Submitted: August 17, 2012
Revised: March 13, 2013
Accepted: March 14, 2013
ARTICLE
Citrate-Capped Gold Nanoparticles asColorimetric Reagent for Copper(II) Ions
by Alan Rodelle M. Salcedo* and Fortunato B. Sevilla III
Research Center for the Natural and Applied Sciences
University of Santo Tomas, España, Manila, Philippines
University of Santo Tomas, España, Manila, Philippines
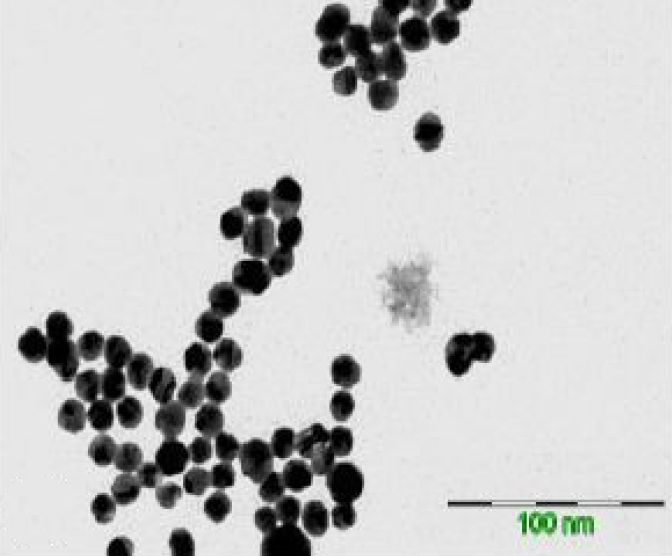
Citrate-capped gold nanoparticles were prepared bychemical reduction of HAuCl4 using citrate as thereducing agent and stabilizer. The synthesizednanoparticles showed an intense localized surfaceplasmon resonance absorption band at 520nm.Uniformly distributed spherical gold nanoparticles with averageparticle diameter size of 12.2±1.65nm were verified throughTransmission Electron Microscopy (TEM) analysis. The stabilityof the gold nanoparticles was affected by pH and Cu2+.Aggregates are formed at pH < 3 due to surface chargeneutralization of the citrate-capped gold nanoparticles. WithCu2+, aggregation is being initiated at a certain concentration.Visual observations, UV-Vis spectroscopy and TEM analysiswere used to characterize the aggregated system. At optimumparameters (i.e., wavelength, reaction time, and pH), acalibration curve for Cu2+ determination was constructed. Thecalibration curve has good linearity (r2=0.9822) and sensitivityof 0.0036 A.U./mM for the linear range from 2.5mM to 100mM.Reproducible absorbance readings (r.s.d.= 1.56-6.15%, n=6) forthe linear concentration range of 2.5mM to 100mM wereobtained. The detection limit for Cu2+ determination method is5.0 mM.
© 2024 SciEnggJ
Philippine-American Academy of Science and Engineering